|
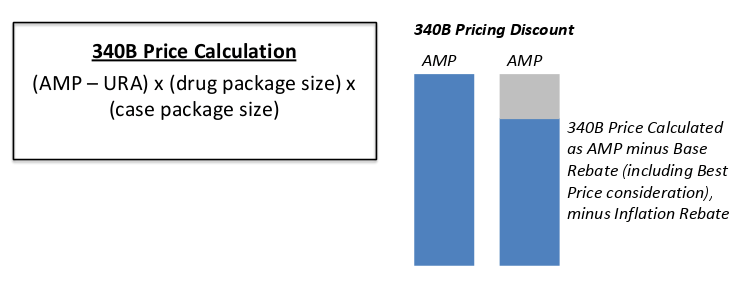
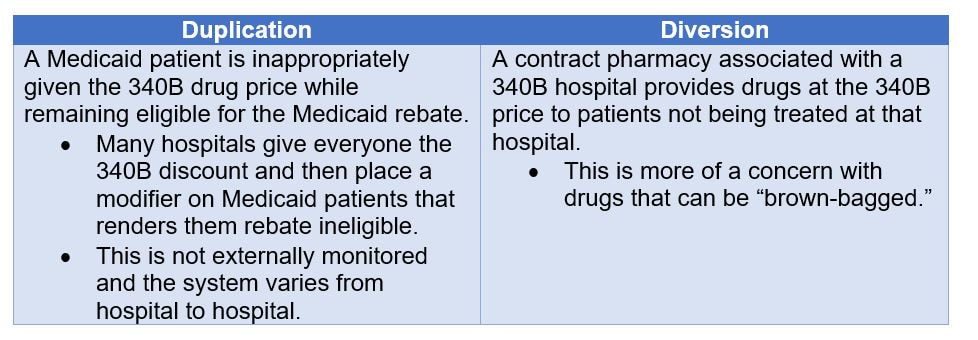
What is 340B? In 1992, the 340B Drug Discount Program was introduced requiring drug manufacturers to provide outpatient drugs to eligible health care organizations and covered entities at significantly reduced prices. The intent of the program is to allow covered entities to stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services. Pharmaceutical manufacturers agree to charge a 340B ceiling price to covered entities by signing a Pharmaceutical Pricing Agreement (PPA) with the secretary of Health and Human Services. As of 2018, six categories of hospitals are eligible to participate in the 340B program. This includes disproportionate share hospitals (DSH), children’s hospitals, cancer hospitals, sole community hospitals, rural referral centers, and critical access hospital. What does 340B mean for drug manufacturers? 340B can result in significantly lower revenue for drug manufacturers as they must discount their drugs when they sell to these covered entities. The drug is discounted through a 340B Price Calculation as follows: Calculated after “two quarter lag”: Q1 sales transactions è Q2 AMP, Best Price, 340B Price Calculated è Q3 340B price becomes effective based on Q1 Sales Orphan Exemption For rural referral centers, sole community hospitals, critical access hospitals, and free-standing cancer hospitals participating in the 340B Program, the term "covered outpatient drug" does not include a drug designated by the Secretary under section 526 of the Federal Food, Drug, and Cosmetic Act for a rare disease or condition. Thus, manufacturers are not required to provide these covered entities orphan drugs under the 340B Program. However, there are relatively few of these rural referral centers. Drug manufacturers may think that their drug will not be subjected to 340B price discounts if it is an orphan, but manufacturers must be aware that the exemption only refers to these few covered entities. In the majority of cases, the drug will be dispensed from a DSH and the 340B discount will still apply. As a measure of good will, manufacturers sometimes choose to voluntarily provide discounts on orphan drugs to the ineligible covered entities. For example, Janssen Pharmaceuticals is offering rural referral centers discounts on its orphan drug Remicade, which is used to treat various autoimmune disorders. Off-Label Orphan Drug Use The Health Resources and Services Administration originally established an interpretive rule that the 340B Orphan Drug Exemption applies only when the drug is used for the orphan indication. Pharmaceuticals Research and Manufacturers of America (PhRMA) challenged this decision in court. In 2015, the U.S. District Court consequently struck down HRSA’s interpretive rule since it was contrary to the language of the 340B statute. Bipartisan legislation was introduced in 2016, called the Closing Loopholes for Orphan Drugs Act, which would limit the 340B drug pricing program’s orphan drug exclusion to apply only when the drug is used for the rare disease indication. The legislation was introduced in the House of Representatives but no further actions were made. The bill was once again introduced in the House in 2017, but no further actions were made. Despite legislative efforts to discount orphan drugs for off-label use, the 2015 court ruling still stands that the orphan drug exemption applies regardless if the drug is used for the orphan indication or not. Again, it is important to note that the orphan drug exemption still applies to only a few covered entities. For most cases, the 340B discount will apply to orphan drugs. Recent CMS Changes to 340B Reimbursement The Center for Medicare and Medicaid Services recently changed the amount that hospitals are reimbursed for 340B discounted drugs. Medicare Part B reimbursement was previously set at Average Sales Price (ASP) plus 6%. Effective January 2018, Part B reimbursement for 340B drugs is ASP minus 22.5%. Drugs which are on pass-through status and vaccines are excluded from the reimbursement reduction. The reimbursement change is applicable to the following covered entities: hospitals, Community Mental Health Centers, and Ambulatory Surgical Centers. The reimbursement reduction would affect orphan drugs that are used off-label as well. Drug Manufacturers’ Concerns Major concerns for manufacturers around 340B pricing is that ineligible patients receive the discount. This can happen via duplication or diversion. Here at BluePrint Orphan we have extensive experience in navigating 340B legislation and have helped many drug companies insure against inappropriate drug diversion. Contact us via info@blueprintorphan.com for more information.
Sources: https://www.healthlawpolicymatters.com/2017/07/17/six-questions-answers-cms-recommended-changes-340b-medicare-reimbursement/ http://340binformed.org/2013/07/new-orphan-drug-rule-makes-sense/ https://www.gpo.gov/fdsys/pkg/FR-2013-07-23/pdf/2013-17547.pdf Info on 340B and Off-Label Orphan Drug Use http://www.klgates.com/340b-orphan-drug-interpretive-rule-struck-down-by-dc-district-court-hhs-and-hrsa-lose-in-second-round-of-litigation-over-340b-orphan-drug-rules-10-22-2015/#_ftn16 https://cases.justia.com/federal/district-courts/district-of-columbia/dcdce/1:2014cv01685/168424/29/0.pdf?ts=1444911425 http://www.modernhealthcare.com/article/20160928/NEWS/160929885 https://www.hrsa.gov/opa/program-requirements/orphan-drug-exclusion/index.html |
Archives
May 2020
Topics
All
|